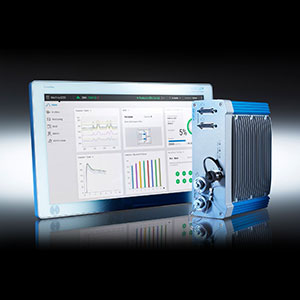
Kistler Group Product
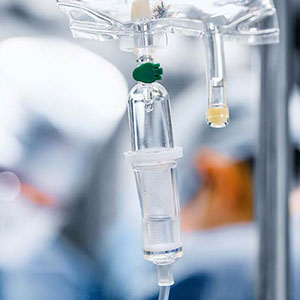
Kistler: your partner in the medical devices market – today and tomorrow
Manufacturers of medical devices have to meet demanding market requirements. They have to comply with large numbers of national and international standards in order to guarantee a product’s safety and quality. Kistler is present throughout the world with a comprehensive portfolio of solutions for sensors and process monitoring systems: your ideal partner in every phase of medical device manufacturing – from the design concept, product development and qualification phases all the way through to production.
Are you interested in achieving zero-defect production, 100% process transparency and enhanced process stability through automated monitoring of process limits? Are you aiming for product traceability with reduced outlay on documentation in your manufacturing facility? The experts at Kistler are always on hand to help you maintain your successful presence in the highly regulated medical market!
The right solution for every application
No matter which application area your medical device is intended for: Kistler supplies solutions precisely tailored to your objectives – solutions that deliver enhanced process transparency and end-to-end documentation.
Maximum quality – from the produced single components through to final acceptance
汇率政策Kistler系统创造条件uring medical devices of impeccable quality – equipping companies to meet future challenges and enhancing patients quality of life through safer products.
Production
Cavity pressure measurement makes it possible to decide whether a produced part is good or bad while the injection molding process is still under way. Kistler’s process monitoring and control system ComoNeo analyses obtained cavity pressure data in real- time.
Assembly
In processes where various components are assembled to produce a medical device, different Kistler sensors are deployed – as appropriate to the application area – to ensure that the product is assembled correctly.
Function test
All the functions have to be verified to ensure that the medical device can be used with absolute safety. On the insulin pen, for example, Kistler sensors test the spring force of the triggering mechanism to prevent incorrect dosage of the medication.
Documentation and data storage
Measured values and obtained process data in the preceding steps are processed as appropriate and made available to the customer. In conjunction with a direct manipulation-proof connection to the customer’s quality database, Kistler technology ensures that all quality-related data is stored securely.
Calibration service
Kistler offers its customers a special service so they can efficiently meet the strict requirements for the manufacture of medical devices: the small force and torque sensors used for these applications can be calibrated directly on-sight. This minimizes costly machine downtime and also eliminates complex acceptance procedures for the plant after the sensors have been calibrated.
Compliance with national and international standards and regulations is the essential and fundamental requirement for the manufacture of products in the medical technology sector.
规定
- EU: MDR
- USA: FDA
- 820年美国食品药品管理局21日
- Country-specific regulations
Good Manufacturing Practice (GMP) Standards
- ISO 9001
- ISO 13485
Click herefor more details on Kistler Medical Device Manufacturing.